|
Electrochromics
  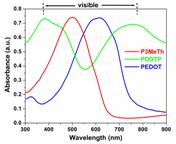
The ability to have three complementary colors, red, green and blue (RGB) constitutes an important step
forward to the use of conducting polymers in polymeric electrochromic
devices (PECDs). Although many red and blue
colored polymers in their neutral form have been reported, no green colored
conducting polymer was reported to date because of the difficulty to obtain
the absorptions required in the visible to reflect the color
green. Here we report the electrochemical and optical properties of
the first electrochemically prepared, neutral, green-colored conjugated
polymer. The extreme stability of this polymer after 10,000 double
potential steps makes it the best, and only, candidate for completing the
third leg of color space for polymeric electrochromics.
Read more… Angew. Chem. Int. Ed., 2004, 43 (12), 1498.
Additive
primary color-space was completed by the discovery of the first green
polymeric electrochromic. Mixing any two of the
three additive primary colors (RGB) in various oxidation levels can produce thousands of
colors resulting from possible tones of these polymers at different
oxidation states. We believe in that the completion of the three legs of colour-space with the discovery of the first green
polymeric electrochromic opened the PECD
era.
Read
more…
Advanced Materials (2004, vol. 16, issue 21, pp.
1905)
Journal of Materials
Chemistry (2005, vol. 1, pp. 20)
The
insertion of a methoxy ethylhexyloxy
benzene ring between two EDOT monomers results in a decrease in wavelength
of the neutral polymer’s p-p* transition. As a
result, another absorption band of stable polarons
arises at the edge of the visible region, producing both a cathodically and an anodically
colored polymer. The P(BEDOT-MEHB) films exhibited
a coloration efficiency value as high as 680 cm2/C at 535 nm and
-360 cm2/C at 760 nm. The coloration efficiency at 535 nm (with
an additional anodic coloration at 760 nm) is almost 4 times higher than
PEDOT and much higher than any other system reported to date. Moreover, the
steric bulk of the methoxy
ethylhexyloxy benzene between EDOT units on the
polymer backbone improves the facility of counter ions injection/rejection,
generating higher doping levels than PEDOT at the same optical densities.
As a material, P(BEDOT-MEHB) is an excellent
candidate for electrochromic displays, exhibiting
very well defined electrochemistry, high robustness to overoxidation
and long term switching stability. Other features are transparency in the
fully oxidized state and high contrast ratio and multi-coloration with very
high coloration efficiencies at two different wavelengths in the visible
region.
Refrences
“A
Red, Green and Blue (RGB) Polymeric Electrochromic
Device (PECD): The Dawning of the PECD Era”, G.
Sonmez, C.K.F. Shen, Y. Rubin, F. Wudl,
Angew. Chem. Int. Ed., 43, 1498
(2004).
“Red,
Green, and Blue Colors in Polymeric Electrochromics”, G. Sonmez, H.B.
Sonmez, C.K.F. Shen, Adv. Mater., 16, 1905
(2004).
“Completion of the Three Primary Colours:
The Final Step Toward Plastic Displays”, G. Sonmez, F. Wudl, J. Mater. Chem., 15, 20 (2005).
“A
Processable Green Polymeric Electrochromic”,
G. Sonmez, H.B. Sonmez, C.K.F. Shen, R. Jost, Y. Rubin, F. Wudl, Macromolecules,
38, 669 (2005).
“Organic
Polymeric Electrochromic Devices: Polychromism with Very High Coloration
Efficiency”, G. Sonmez, H. Meng, F. Wudl, Chem. Mater., 16, 574 (2004).
|